views
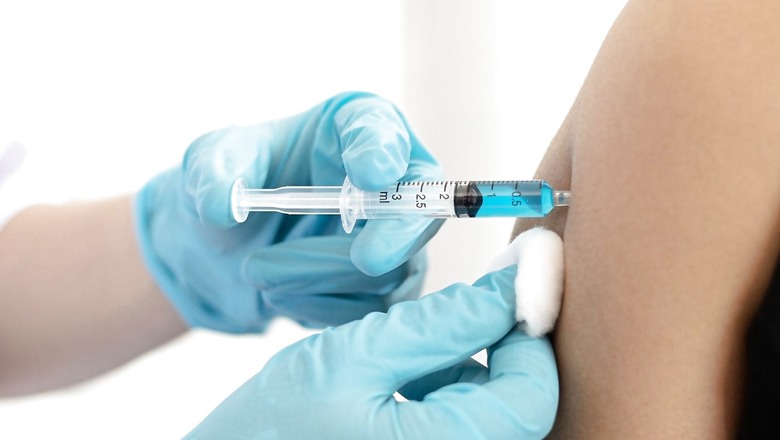
The Serum Institute of India has submitted an application for ‘market authorisation’ of Covishield to the subject expert committee (SEC) of the Drugs Controller General of India (DCGI). The committee will meet today to discuss the company’s submission.
Currently, Covishield is being administered in the country in emergency-use authorisation mode. The vaccine was approved on January 4, 2021, in EUA and has been administered to over 104 crore people in the country.
In addition, the vaccine has also been exported to neighbouring countries, including Bangladesh, Nepal and Myanmar.
EUA does not represent approval of a vaccine in the full statutory term but authorises the use of an unapproved product or unapproved use of an approved product in a declared state of emergency such as a pandemic. A market authorisation label for a vaccine means it can be authorised for use without reservations or conditions.
Bharat Biotech applies for nod to conduct clinical trials on pregnant women
India had allowed Covid-19 vaccines for pregnant women in July this year based on a recommendation from the National Technical Advisory Group on immunisation but no trials were conducted on pregnant women to determine the risk-benefit assessment.
Bharat Biotech has submitted its application to the SEC to conduct phase 2/3 clinical trials on pregnant women.
The committee will consider the company’s application today.
To date, over 20 lakh doses of Covid-19 vaccines have been administered to pregnant women in the country.
Read all the Latest India News here















Comments
0 comment