views
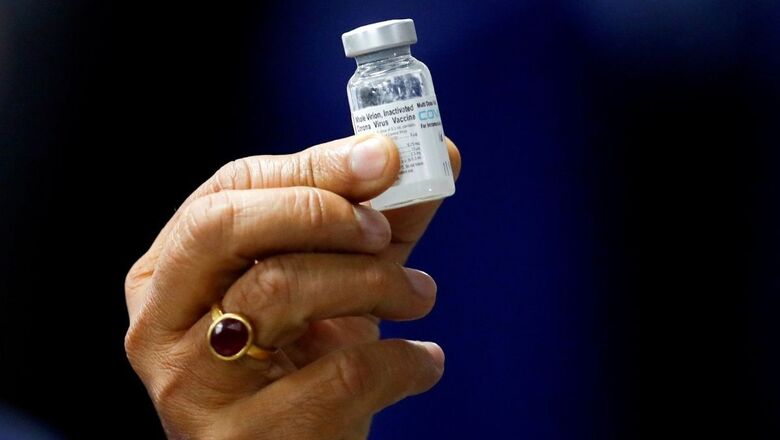
There could be some delay in shipping Covaxin to the seven countries shortlisted to receive the Covid-19 vaccine as a goodwill gesture, as the government is waiting for approvals from their respective regulatory authorities, sources have told News18.
On January 16, the Indian government decided to donate 8.1 lakh doses of India’s indigenous vaccine Covaxin to a select few countries, in what has been dubbed as Covid diplomacy. The Ministry of External Affairs, it was decided, would make the procurement after January 22.
On January 18, a high-level meeting was held in the health ministry with foreign minister S Jaishankar, health minister Dr Harshvardhan and MoS Pharmaceuticals Mansukh Mandaviya. Serum Institute of India was represented by its CEO Adar Poonawalla and Bharat Biotech by CMD Dr Krishna Ella.
Also Read | Amid Concerns Among Citizens over Vaccine Safety, Lancet Endorses Covaxin Phase I Trial Results
It was decided at this meeting that doses of Covaxin would be sent to Myanmar, Mongolia, Oman, Bahrain, Philipines, Maldives and Mauritius. But there seems to be a hurdle as Covaxin is being administered in India in ‘clinical trial mode’ and its efficacy data is still awaited.
Bharat Biotech had completed Phase-2 trials, and started a Phase-3 trial for Covaxin in India in November. Efficacy data from this study is expected by March.
Sources have told News18 that the required dossiers have been sent to each of these countries after which a nod from their respective drug regulators would be sought.
Given the exceptional circumstances under which Bharat Biotech’s Covaxin got accelerated use approval seems to have led to some amount of hesitancy among citizens who fear taking the shot.
However, the developers of the vaccine heaved a huge sigh of relief on Friday after The Lancet Infectious Disease journal published a study that said that Covaxin showed enhanced immune response without any serious side effects in the participants enrolled for the phase 1 trials.
Covid-19: Rising Reluctance to Take the Shot Latest Challenge Before Centre
Nevertheless, with the efficacy data yet to be generated and the term ‘Clinical Trial Mode’ still associated with the vaccine, apprehension among citizens is high, said sources within the Hyderabad-based pharma company.
Covishield, on the other hand, has been sent off to a host of countries as a goodwill gesture. Besides commercial supplies, India has given free doses of Covishield to Maldives, Mauritius, Bhutan, Nepal and Bangladesh. Doses have been sent to Seychelles and Myanmar as well.
Read all the Latest News, Breaking News and Coronavirus News here
















Comments
0 comment