views
X
Research source
Measuring Steady-State Samples
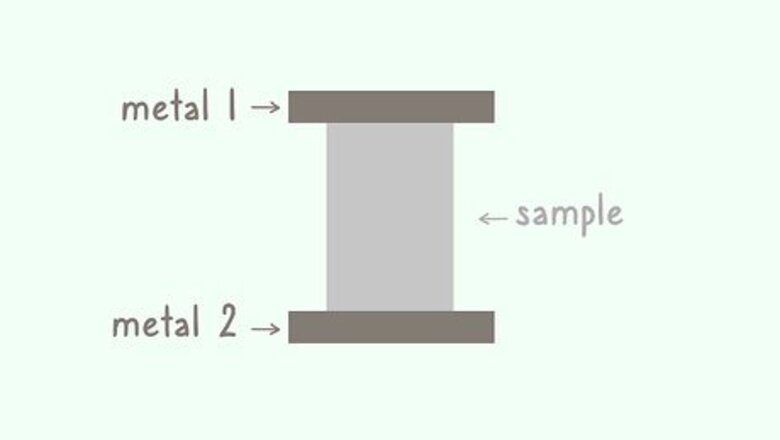
Place the sample in between 2 plates of metal. The best way to measure a steady-state sample is with the hot plate method. If your sample is flat and mostly rectangular, place it between 2 metal plates in the lab. Make sure you have enough space to cool and heat each plate. Steady-state material does not change even when it goes through a transformation or change. If you add a change-agent to a chemical mix but it retains its properties, it is a steady-state material.
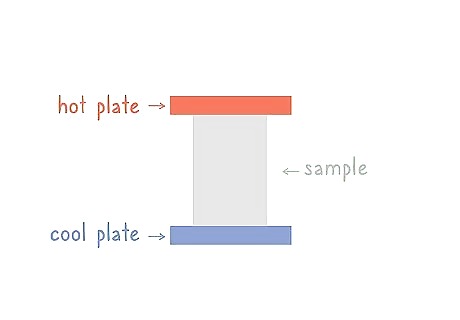
Heat the top plate and cool the bottom plate until the temperatures are steady. Use a heating apparatus to heat the top plate and a cooling apparatus to cool the bottom plate. You can set a certain temperature for each plate or just monitor them to see what temperature they arrive at. It could take up to 10 minutes for your temperatures to become steady.
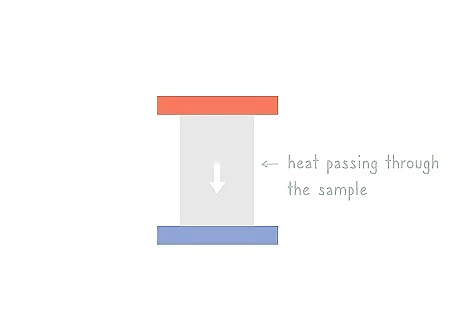
Monitor the amount of heat passing through the sample. Thermal conductivity is the amount of heat that is lost over time. Use a thermometer to measure the amount of heat passing through the sample from the warm side to the cool side to get your thermal conductivity constant. Plug this into your thermal conductivity equation. Put your thermometer in an unobtrusive area of your sample.
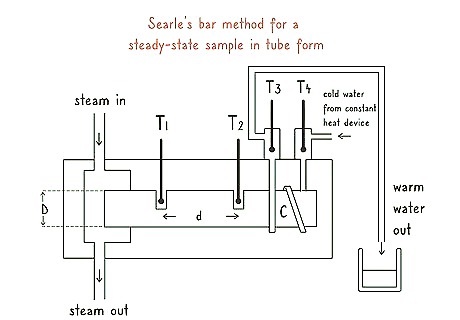
Apply Searle’s bar method for a steady-state sample in tube form. Use a Searle’s bar apparatus to test the rate of thermal conductivity if your sample is in a pipe, like copper. Put your sample into the center of the apparatus. Place the steam end of the apparatus into a sink. Adjust the head of the apparatus to ensure a steady flow of water over your sample. Measure the temperature of the water as it exits the apparatus.Tip: Searlee’s bar apparatus can be difficult to use if you do not have experience doing so. Get an experienced lab technician to help you set up the apparatus if you need to.
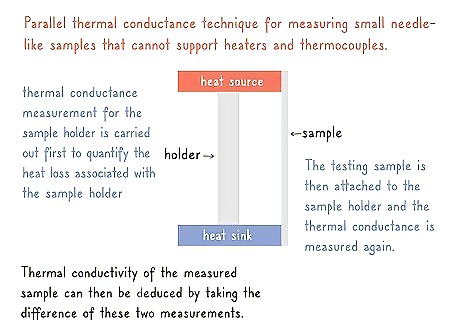
Test a small, thin sample with a parallel thermal conductance. Thin samples can’t handle as much pressure as thick, cylindrical samples can. Place your sample on a stage in between a heat source and a heat sink. Measure the heat lost over time. Then, measure the stage to test its thermal conductivity. Subtract the stage’s conductivity from the sample’s conductivity.
Measuring Nonsteady-State Samples
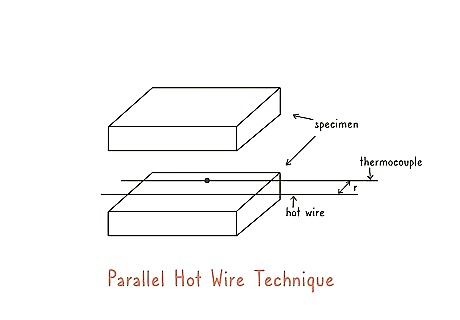
Insert a hot wire into the middle of your sample. Nonsteady-state samples are much more likely to be foams or gels that can have a wire inserted into them. Heat a wire and note the temperature that it starts at. Insert the wire into the middle of your sample where it is the thickest. The wire is fairly intrusive, so it can’t be used on solid samples. Nonsteady-state materials do change when they go through a transformation or change.
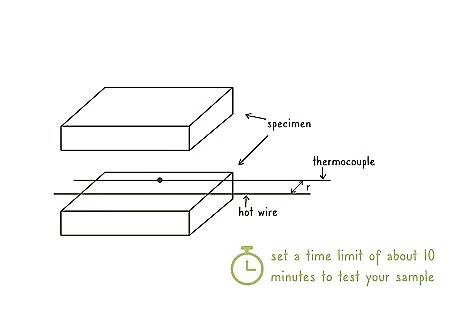
Monitor the temperature change in the wire over time. Set a time limit of about 10 minutes to test your sample. Monitor the change in the temperature of the wire while it is inside of your sample.
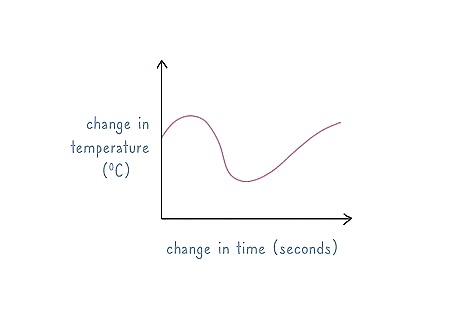
Plot the temperature change on a graph. Use the change in time on one axis and the temperature change on another axis. Use the temperature changes of the wire to calculate thermal conductivity by comparing it to the logarithm of time.Tip: You can modify this wire test to instead be supported on a backing. This way, it doesn’t actually have to penetrate the sample itself.
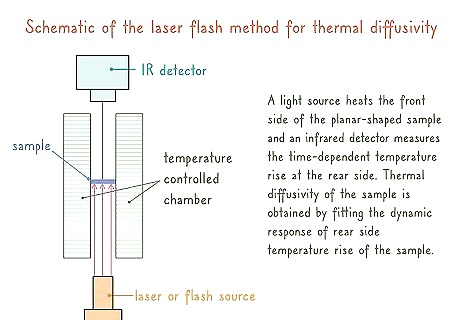
Monitor a laser flash for a quick way to test nonsteady-states. Use a laser flash to quickly deliver a short pulse of heat to your sample. Monitor your infrared scanner to identify the change in temperature over time throughout the sample.
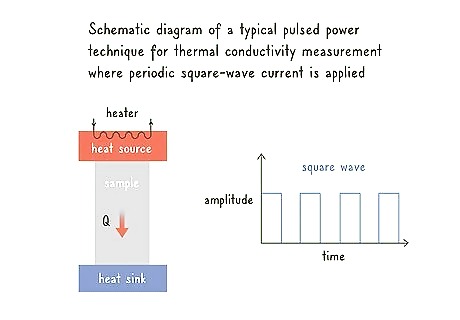
Measure thermal conductivity and thermal power with pulsed power. Hold your cylindrical or triangular sample in between a heat source and a heat sink. Use a square-wave or a sinusoidal wave from your heat source to send an electric current into your sample. Measure the heat lost and the electric current over time.
Using an Equation
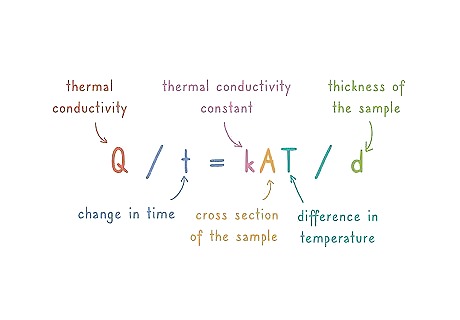
Write down the equation for thermal conductivity: Q / t = kAT / d. In order to measure thermal conductivity, you need to account for all variables that could affect heat loss or gain. Time, thickness of the sample, thermal conduction constant, and temperature of the test are all taken into account when solving for thermal conductivity. In the equation, “Q” stands for the amount of heat transferred over time, or the thermal conductivity. “t” denotes the change in time. ”k” denotes the thermal conductivity constant. ”A” denotes the cross section of the sample that is conducting heat. ”T” is the difference in temperature from the cold side of the sample to the hot side of the sample. ”d” denotes the thickness of the sample.
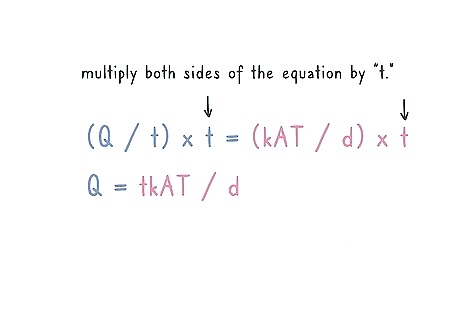
Multiply both sides of the equation by “t.” In order to solve your equation, “Q” needs to be isolated. Multiply your equation by “t” so that “Q” stands on its own on the left of the equal sign. For example: (Q / t) x t = (kAT / d) x t That makes the equation: Q = tkAT / d
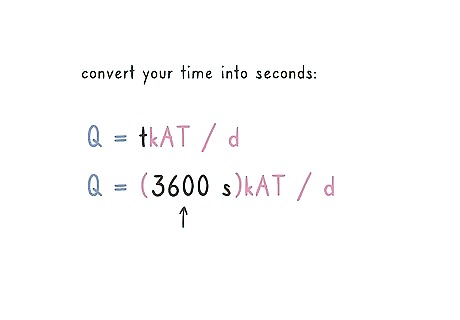
Convert your time into seconds and plug it into the equation. Your problem or experiment most likely gave you a time period in minutes or even hours. If your time is in minutes, multiply the minutes by 60 to get seconds. If your time is in hours, multiply the time by 3600 to get seconds. Plug your seconds into the “T” of the equation. For example, if you have 30 minutes, take 30 x 60 = 1800 seconds. If you have 1 hour, multiply 1 x 3600 = 3600 seconds. Your equation should read: Q = (3600 s)kAT / d
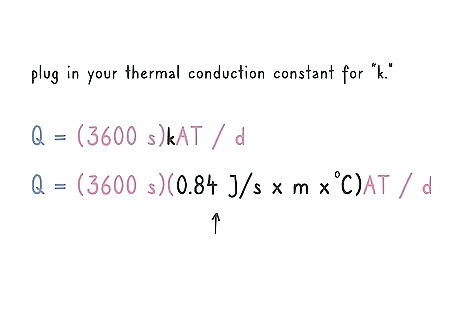
Plug in your thermal conduction constant for “k.” The temperature that your sample averaged out to be at is usually given in a fraction of joules per second per meter per degree. Substitute your thermal constant for the “k” in your equation. For example: Q = (3600 s)(0.84 J/s x m x °C)AT / d

Multiply height x width of your sample and plug it into the “A.” Get the area of your sample by multiplying the height and width of your sample. If your sample was a liquid, use volume instead of area. Plug the area into the “A” of your equation. Make sure your area is in meters squared. For example:
If the sample is 0.65 m tall and 1.25 m wide, multiply 0.65 x 1.25 to get 0.8125 m
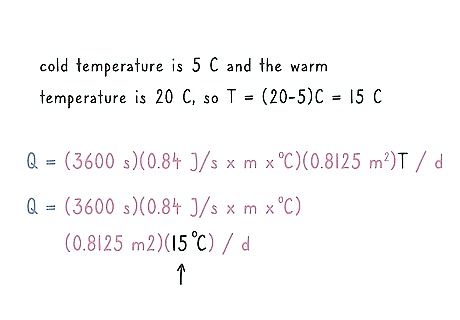
Subtract the cold from the hot temperature and use it for “T.” Use the cold temperature and the hot temperature to figure out the change in temperature overall. Take the cold temperature away from the warm temperature to figure out the total change. Keep the units the same when you subtract. If the cold temperature is 5 °C (41 °F) and the warm temperature is 20 °C (68 °F), subtract 20 °C - 5 °C = 15 C. Q = (3600 s)(0.84 J/s x m x °C)(0.8125 m)(15 °C) / d

Insert the thickness of your sample for “d.” The total thickness affects the rate at which heat will leave your sample. Convert the thickness of your sample into meters and then plug it in for “d” in your equation.Tip: If your sample thickness is in centimeters, divide it by it by 100 to get meters. For example, 2 cm / 100 = 0.02 m. Q = (3600 s)(0.84 J/s x m x °C)(0.8125 m)(15 °C) / 0.02 m
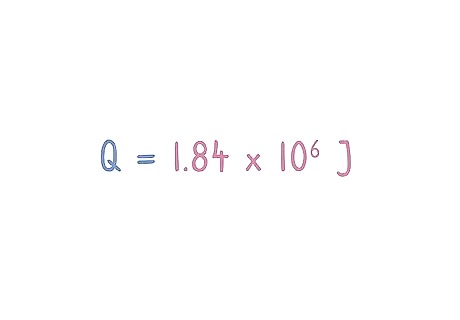
Calculate your equation to get joules of heat. Follow the order of operations to complete your equation. Cancel out every unit besides joules as you follow your steps. If your number is larger than 2 decimal points long, use significant figures to complete it. Q = 1.84 x 10 J


















Comments
0 comment