views
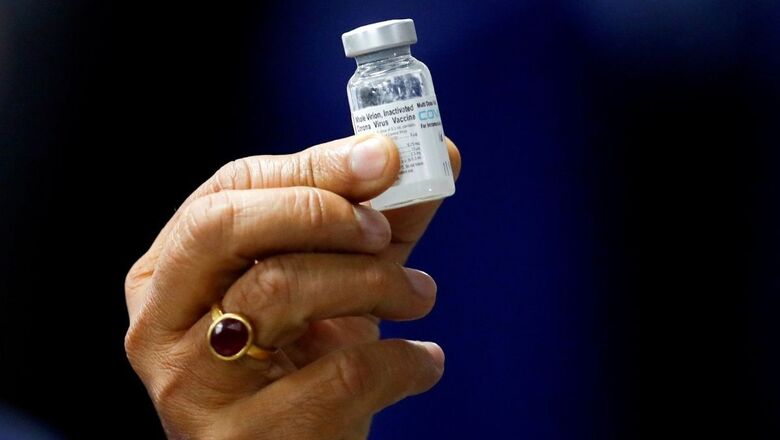
The Drugs Controller General of India (DCGI) approved Phase II/III clinical trial of COVAXIN in the age group of 2 to 18 years on Thursday. Bharat Biotech is manufacturing India’s indigenous vaccine Covaxin and has conducted trials in 525 healthy volunteers.
During the phase two study of Covaxin, the 380 participants included people aged between 12-65 years. The vaccine led to tolerable safety outcomes and enhanced humoral and cell-mediated immune responses. In phase three trials, only adults were enrolled.
The National Regulator of the country, the Drugs Controller General of India (DCGI), after careful examination, has accepted the recommendation of Subject Expert Committee (SEC) and accorded permission to conduct the Phase II/III clinical trial of Covaxin (COVID vaccine) in the age group 2 to 18 years, to its manufacturer Bharat Biotech Ltd on May 12.
M/s Bharat Biotech International Ltd., Hyderabad (BBIL) had proposed to carry out a Phase- II/III clinical trial of Covaxin in the age group of 2 to 18 years.
In the trial, the vaccine will be given by intramuscular route in two doses at day 0 and day 28. As rapid regulatory response, the proposal was deliberated in Subject Expert Committee (SEC) (COVID-19) on May 11. The Committee after detailed deliberation recommended for grant of permission to conduct proposed Phase II/III clinical trial to certain conditions.
Bharat Biotech which conducted clinical trials on children aged between 2 and 12 years of age, the jab will be made available for this age group soon.
This comes at a time when US gave nod to Pfizer vaccine for children between 12 to 15 years of age. Vaccine for all, including children, is being seen as an important step the smash the rising graph of Covid cases and disparity between vaccinated and non-vaccinated population to return to normalcy.
Covaxin and Covishield have been made available for people above the age of 18, while several vaccination centres are administering doses only to people above the age of 45 in view of shortage.
The US on Monday authorised the Pfizer-BioNTech Covid-19 vaccine for children aged 12 to 15 years old. The US Food and Drug Administration previously had granted an emergency use authorisation for the jab to individuals aged 16 and older.
The virus is still surging in many countries and the pandemic has killed close to 3.3 million people worldwide since late 2019, upending normal life and causing global economic chaos.
Rapid vaccination programmes, however, have allowed a number of wealthy nations to start taking steps towards normality.
The head of the European Medicines Agency said on Monday that BioNTech/Pfizer’s jab against Covid-19 soon could be approved for 12- to 15-year-olds in the EU, as well, perhaps as early as this month.
Read all the Latest News, Breaking News and Coronavirus News here. Follow us on Facebook, Twitter and Telegram.
















Comments
0 comment