views
New Delhi: In an interim relief for pharma companies, the Delhi High Court on Monday stayed the ban on 344 drugs till March 28. The court will hear all matters related to the ban on drugs announced by the Union Health Ministry as some parties are yet to file their reply.
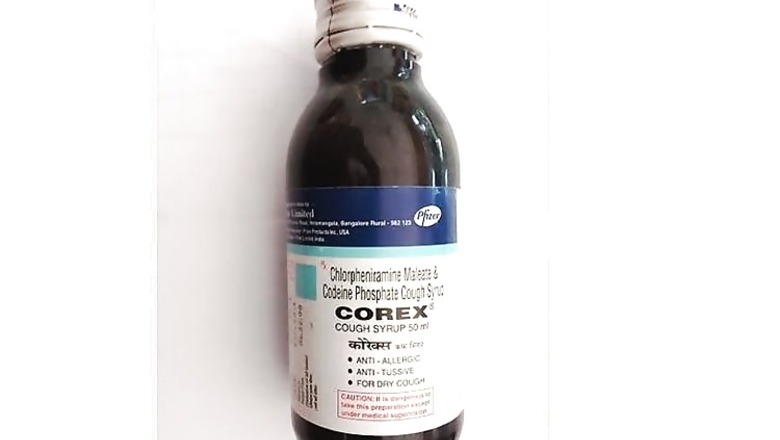
Some of the popular drugs that were banned include cough syrups like D'Cold, Phensedyl, Benadryl and Corex, Nimesulide and Vicks Action 500 Extra. The government announced the ban on 344 fixed drug combinations on March 12 sending the pharma industry into panic.
The court is hearing the pleas by over 30 pharma majors including Pfizer, Glenmark, Procter and Gamble (P&G) and Cipla, challenging the government’s decision to ban sale of some of their fixed dose combination medicines.
Justice Rajiv Sahai Endlaw had earlier stayed operation of the government’s notification till March 21 and directed the Centre not to take coercive steps against these companies.
Initially the interim relief was given on March 14 to Pfizer’s cough syrup Corex. Later the same was granted to over 30 companies, which also included Glaxo Smithkline, Reckitt Benckiser, Abbott Healthcare, Piramal, Lupin, Mankind Pharma and Wockhardt.
Apart from them, the other companies which have moved thehigh court are Alembic Pharmaceutical, Ajanta Pharma, Macleods Pharmaceuticals, Dr Reddy’s, Laborate Pharmaceuticals, Alkem Laboratories, Khandelwal Laboratories Pvt Ltd, Micro Lab Ltd, FDC Ltd, Coral Laboratories Ltd and Eris Lifesciences Pvt Ltd.
Some of the well-known medicines on which the ban on sale has been lifted include Pfizer’s Corex cough syrup, Glaxo’s Piriton expectorant, P&G’s Vicks Action 500 extra, Reckitt’s D’Cold, Piramal’s Saridon, Glenmark’s Ascoril and Alex cough syrups, Abbott’s Phensedyl cough syrup and Alembic’s Glycodin cough syrup.
In the petitions of some of the companies, also including Laboratories Griffon, Centaur Pharmaceuticals, Shreya Life Sciences, Geno Pharmaceuticals and Unichem Laboratories, where they have obtained licence from state authorities for the purpose of manufacturing, marketing, distribution and sale of the fixed dose combination drugs, the court passed a different order.
In such cases, the court while staying the effect of the notification, also allowed the government to take action under any other available law for sale of those drugs which are made, marketed, distributed or sold on licence from state authorities instead of Drugs Controller General of India (DCGI).
This order was passed after the Centre on March 18 said under the Drugs and Cosmetics Act and Rules, the companies after 1988 had to obtain licence from DCGI and those which have got licence from state bodies are not entitled for any interim relief.
In their pleas, the healthcare companies sought quashing of the government’s March 10 notification banning over 300 fixed dose combination drugs, including cough syrup compositions on the ground that they involve “risk” to humans and safer alternatives were available.
The companies have contended that the decision was taken by the government without issuing them a show cause notice or granting them a hearing.
They have alleged that the notification was silent on the aspect as to which expert committee was appointed by Health Ministry to examine the safety and deficiency of the fixed dose combination drugs.
As per the notification, “On the basis of recommendations of an expert committee, the central government is satisfied that it is necessary and expedient in public interest to regulate by way of prohibition of manufacture for sale, sale and distribution for human use of said drugs in the country.”
(With inputs from PTI)



















Comments
0 comment