views
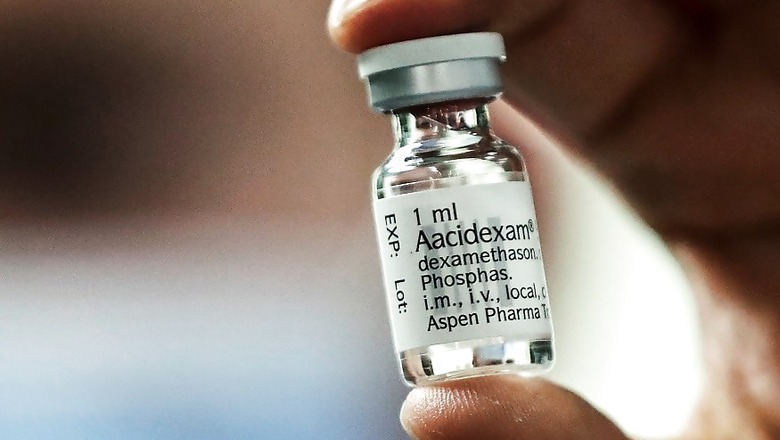
Dexamethasone, an inexpensive and widely used steroid, was included in the treatment protocols for Covid-19 patients in moderate to severe stages of illness among other therapeutic measures by the Union health ministry on Saturday.
According to WHO, the corticosteroid dexamethasone was tested in hospitalised patients with Covid-19 in the United Kingdom's national clinical trial 'RECOVERY' and was found to have benefits for critically ill patients.
According to preliminary findings shared with WHO, for patients on ventilators, the treatment was shown to reduce mortality by about one-third, and for patients requiring only oxygen, mortality was cut by about one-fifth.
The updated protocol includes the advice to use dexamethasone as an alternative choice to methylprednisolone for managing moderate to severe cases of COVID-19. The change has been made after considering the latest available evidence and expert consultation, the ministry said.
According to the revised 'Clinical Management Protocols for COVID-19', dexamethasone which is already used in treating lung infections besides in a wide range of conditions for its anti-inflammatory and immunosuppressant effects can be used as an alternative to methylprednisolone which already existed in the treatment guidelines.
The health ministry on June 13 had also allowed the use of antiviral drug remdesivir for restricted emergency use and off-label application of tocilizumab, a drug that modifies the immune system or its functioning, and convalescent plasma for treating COVID-19 patients in moderate stage of the illness as an investigational therapy.
It also recommended hydroxychloroquine in patients during the early course of the disease and not on critically ill patients. The use of these drugs continues to be included in the revised treatment protocols under the 'investigational therapy'.
The revised treatment protocols for moderate cases advised considering methylprednisolone 0.5 to 1 mg/kg or dexamethasone 0.1 to 0.2 mg/kg for three days, preferably within 48 hours of admission or if oxygen requirement is increasing and if inflammatory markers are increased. The duration of administration should be reviewed as per clinical response.
The revised treatment protocols were issued as India's COVID-19 tally raced past the five-lakh mark on Saturday with the biggest single-day surge of 18,552 cases while the death toll climbed to 15,685 with 384 fatalities, the updated figure at 8 am showed.


















Comments
0 comment