views
Distilling Alcohol From Water
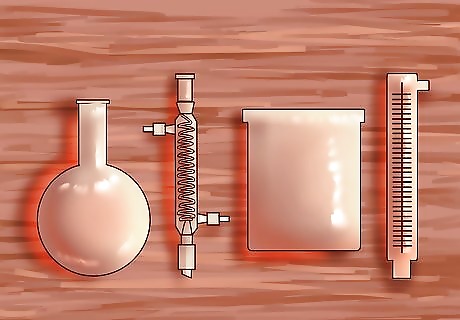
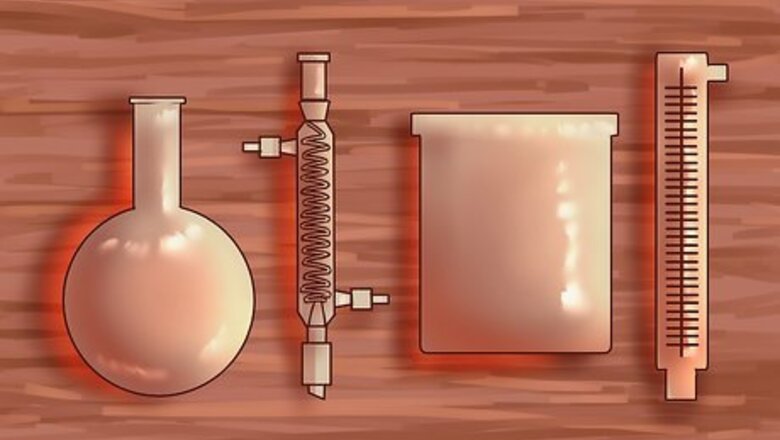
Create a closed system for distillation. The most simple distillation system uses a round-bottomed glass flask (or boiling flask), a condensing unit, and a second glass container for the separated liquid, or distillate. Using a fractional (or fractionating) column inserted between the boiling flask and the condensing unit is recommended for separating alcohol and water. The simple distillation system requires the two liquids have a large difference in boiling points. The simple distillation system uses less heat, and is easier to set up, but provides less accuracy in separating alcohol from water. Another word for the closed distillation system is a still, which is derived from the word distillation.
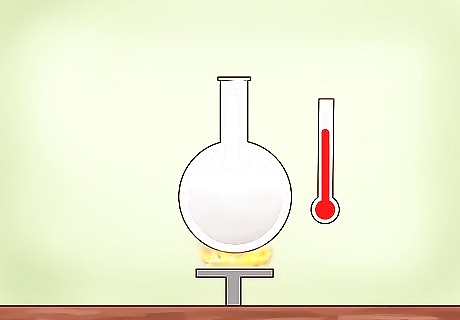
Heat the alcoholic water mix in the round-bottomed flask to 80 °C (176 °F). The boiling point of water is 100 °C (212 °F), and the boiling point of alcohol is 78 °C (172 °F) Celsius. Thus, alcohol evaporates into steam quicker than water. Use a heat source whose temperature can be quickly raised or lowered, such as a heating mantle or bunsen burner, but these may be hard to control the temperature. You can also use a standard propane or electric heating source.
Insert a fractionating column into the mouth of the flask. The fractionating column is a straight glass cylinder lined with metal rings, or glass or plastic beads. These rings or beads help trap the less volatile gases at the lower levels of the column. As the vapor rises from the distilling liquid, only the most volatile liquid rises to the top. In a mixture of alcohol and water, alcohol would make its way to the top ring. Insert a thermometer to gauge the temperature of the gases inside the system.
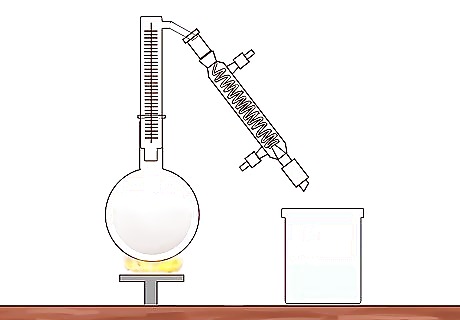
Allow the vapor to cool and condense. As the vapor makes its way into the condensing column, it will be in a cooler setting. Being in this cooler place, it will revert into liquid, i.e., condense. The distillation process goes heating, evaporating, cooling, and finally, condensing. As the vapor condenses to a liquid, it will become heavier. The liquid alcohol will then drop into the collection vessel. The condensing column may be lined with cooling water to speed the process.
Separating Alcohol Through Freezing
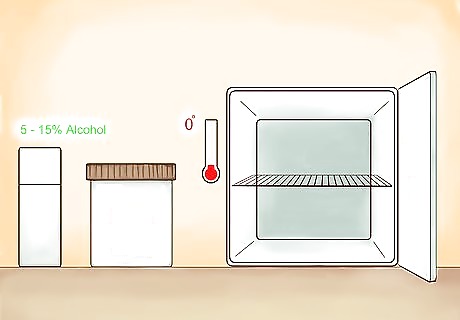
Start with a liquid that is 5%-15% alcohol. You'll need a container that can be safely frozen and thawed, and a place (either a freezer or outdoor temperatures) that are below 0 °C (32 °F). This method relies upon the different freezing temperatures of alcohol and water, much as heat distillation relies upon different boiling temperatures. This is an ancient technique of separating alcohol from water, practiced since the 7th century. Freeze distillation is sometimes known as the Mongolian still.
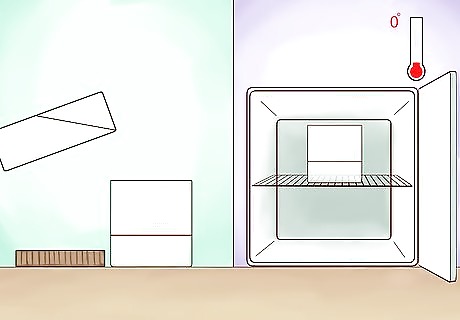
Place the alcoholic liquid into a container. As water expands when it freezes, make sure that your container is large enough to hold the expanded liquid without bursting. The water content of the liquid will expand, but the amount of alcoholic beverage will be much less, due to the extraction of the water. The freezing point of water is 0 °C (32 °F) while the freezing point of alcohol is −114 °C (−173 °F). In other words, alcohol will never freeze under ordinary conditions. Siphon the liquid from the frozen substance once a day. The longer you leave your container in the freezer (or outside), the higher the alcoholic content of your remaining liquid. For larger amounts, use larger containers. Be sure to use food grade plastic containers, as lower quality plastics may contaminate your beverage.
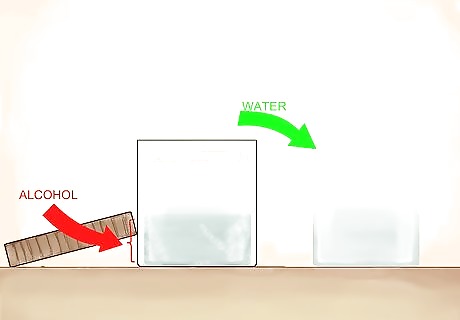
Remove the frozen material from the container. The frozen material will be mostly water, while the alcohol, which has a higher freezing temperature, will be left behind. The remaining liquid will be higher in alcoholic content, though not pure alcohol. It will also have a stronger flavor. For that reason, this is a popular distillation technique with hard apple cider (or apple jack), ale, or beer. The name apple jack comes from the freeze distillation process, which has historically been known as jacking. This method does not allow you to remove impurities like heat distillation would.
"Salting Out" Alcohol From Water

Add salt to isopropyl alcohol to process by azeotropic distillation. This distillation process separates the water from the alcohol by dehydration. Dehydrated isopropyl can be used as fuel, as a removal for fleas and ticks from pets, as an antiseptic for pets or humans, or as a deicer for windshields. Dehydrated isopropyl is an essential part of creating biodiesel fuel. This process is known as extractive distillation.
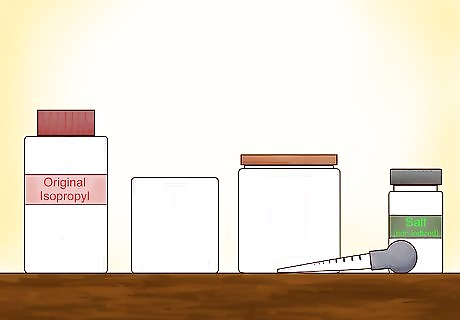
Gather your materials. To separate water from isopropyl alcohol, you'll need the original isopropyl alcohol mix (50% to 70% isopropyl alcohol mix) and a container to hold this liquid when finished, a wide-mouth ⁄2 US gal (1.9 L) glass jar for mixing, 1 pound (450 g) of non-iodized table salt, and a baster with the reduced-size nozzle. Make sure all your materials are clean, including the jars and your baster. Isopropyl alcohol is commonly sold over-the-counter in pharmacies in 16 fl oz (470 ml) bottles. You'll need 32 fl oz (950 ml) for a ⁄2 US gal (1.9 L) glass jar.

Fill the mixing container about 1/4 full of table salt. Make sure you're not using iodized salt or it will contaminate the distillation process. This should be roughly the contents of one standard container of table salt. Use any brand of salt you choose, as long as it's not iodized. You may use any amount of alcohol and salt you like, as long as it follows the ratio of four parts liquid to one part salt.

Add the alcohol to the mixing jar and shake well. Your mixing jar should be about 3/4 full with the isopropyl alcohol and salt mixture. If it's any fuller than that, it may not have room for the expansion that will occur when salt mixes with alcohol. Make sure your lid is well fastened before shaking. Watch to make sure salt is well combined with liquid before ceasing to shake.
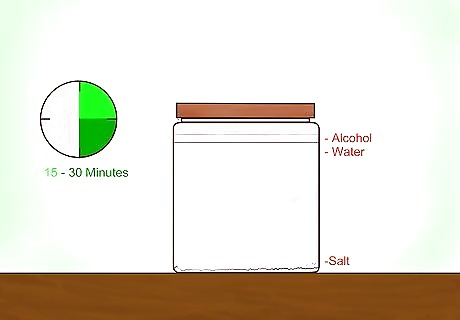
Allow gravity to separate the contents of the mixture. It will take 15-30 minutes for the salt to settle to the bottom of the jar. The liquid rising to the top will be higher in alcohol. This is the dehydrated isopropyl alcohol. Don't allow the two layers to remix This happens because the salt bonds with the water rather than the alcohol bonding with the water. When you open the jar, do so very carefully to prevent excess shaking. Excess shaking will disturb the salty contents at the bottom of the jar and require you to repeat the distillation process.
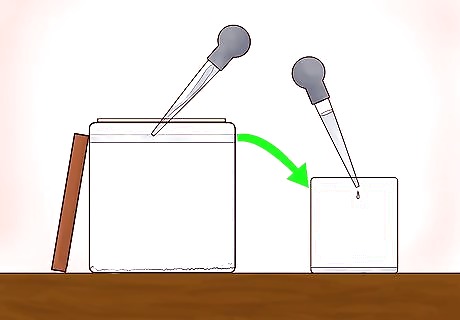
Use the baster to extract the distilled alcohol from the top of the mixing jar. Have your receiving container nearby, already labeled as "distilled isopropyl alcohol." The baster can be used very gently to remove one small bit at a time from the mixing container. Be careful not to shake, pour or tilt the mixing jar as you remove the distilled alcohol.
















Comments
0 comment