views
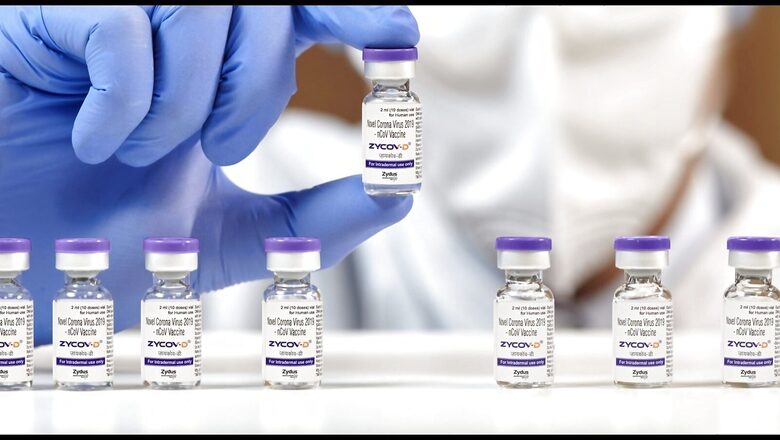
After the first indigenous Covid-19 vaccine, Covaxin, India is all set to roll out its second made-in-India vaccine. ZyCov-D is a needle-free vaccine, unique in nature due to the fact that it has been developed on a plasmid DNA platform. The vaccine will first be administered to adults, and then be rolled out for children aged between 12 and 17. It is also the first vaccine for adolescents in India.
Here’s all you need to know about ZyCov-D, its availability, and how the vaccine works:
What is the ZyCov-D vaccine?
The ZyCov-D vaccine was authorised by Drugs Controller General of India (DCGI) in August. It is a needle-free vaccine administered in three doses, tested at Central Drug Laboratory (CDL) at Kasauli in Himachal Pradesh and manufactured by Cadila Healthcare. The pharmaceutical company has released 2,37,530 doses of the vaccine, which is India’s second indigenous vaccine. It is the first plasmid DNA vaccine in the world for human use.
What is ZyCov-D’s importance in the current Covid-19 scenario?
Amid fear triggered by the Omicron variant of the novel coronavirus, it is important to strengthen India’s vaccine capacity. Another vaccine for the adult population may aid in increasing the pace of vaccination.
An official at Zydus Cadila said the company is “closely monitoring the global data on Omicron”. The official further said the company’s vaccine is based on “plug and play technology” as it can be easily adapted to deal with mutations in the virus. “We need to do an easy tweaking, if the situation demands it.”
Parents can also heave a sigh of relief as they have eagerly awaited Zydus Cadila’s vaccine that has been tested on children aged between 12 and 17. It is the first vaccine for use in adolescents in the age group of 12 to 17 in the country. The central government, though, is yet to come up with guidelines for the vaccine’s administration in children.
How is the vaccine administered?
The three doses of the ZyCoV-D vaccine will be administered on day 0, day 28 and day 56. Each of the three doses will be given in two shots on each arm, right and left. For an individual to be fully vaccinated by ZyCoV-D, one would have to get six shots of the vaccine. Unlike Covid vaccines, such as Covishield and Covaxin, ZyCoV-D comes in a needle-free system. A disposable painless jet applicator is used to administer the dose. The three-dose vaccine will cost Rs 1,128 to the government.
When and where will the vaccine be available?
The vaccine will be available across seven Indian states. But it will only be administered to adults for now. The seven states are Bihar, Jharkhand, Maharashtra, Punjab, Tamil Nadu, Uttar Pradesh and West Bengal. These states have been asked to plan sessions based on the PharmaJet injector and identify vaccinators to be trained for using it for vaccination.
What is a plasmid DNA vaccine? How is it different from other vaccines in India?
The Zydus Cadila shot belongs to a category known as genetic or nucleic acid vaccines. These vaccines work by inserting a piece of the virus’s genetic information into the body to prompt cells to produce a key component of the virus which the immune system then recognises and attacks by producing antibodies.
According to US-based think tank Milken Institute, “DNA-based vaccines work by inserting a genetically engineered blueprint of viral gene(s) into small DNA molecules (called plasmids) for injection into vaccinated people.” Once inside the human body the “cells take in the DNA plasmids and follow their instructions to build viral proteins, which the immune system recognises as foreign, triggering the immune response that protects against the disease”.
ZyCoV-D can be stored at 2-8 degrees Celsius temperatures but it has shown good stability at temperatures of 25 degrees Celsius for at least three months. That would enable easy transportation and storage, unlike mRNA vaccines, which require ultra-cold storage.
EXPLAINED | Shield Against Multiple Variants, Newer Coronaviruses. What 2nd Gen Covid Shots Are Aiming At
Read all the Latest India News here















Comments
0 comment