views
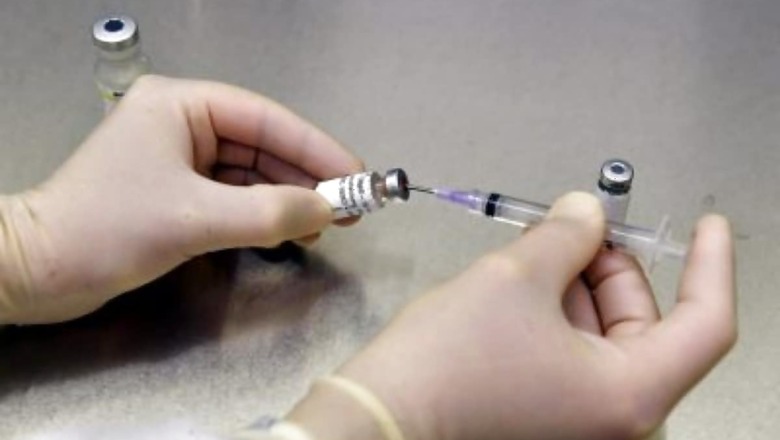
Israel is set to start the second phase of human clinical trials of its COVID-19 vaccine candidate soon following the successful completion of the first phase, the Ministry of Defence (MoD) said. The second phase of the human trial of the vaccine, named Brilife, will involve the participation of approximately 1,000 volunteers. "The Ministry of Health has approved the continuation of clinical trials for the COVID-19 vaccine developed by the Institute for Biological Research (IIBR), following the successful completion of the first phase," the MoD said in a statement.
"During the first phase, no significant side effects were identified, and two expert committees, both internal and external recommended the approval of the second phase. As such, the institute completed all the necessary preparations and is ready for the immediate launch of the second phase," the statement said. The trial will take place at Sheba and Hadassah Medical Centers and will gradually expand to additional medical centres across the country. The first phase of the human trial that kicked off on November 1 included a series of safety tests with the participation of 80 healthy volunteers (aged 18-55), designated by Sheba and Hadassah medical centers.
"The scientists of the IIBR are Israel's 'elite unit,' and have taken on an extremely important task – saving human lives. I see great importance in the development of an Israeli vaccine that will continue to serve Israeli society for years to come," Alternate Prime Minister and Defence Minister, Benny Gantz, was quoted as saying in a press statement from the MoD. The second phase of the trial to be conducted over a period of several months will include extensive safety tests with the participation of 1,000 healthy volunteers aged 18 and over. The scientists aim to complete vaccine safety precautions, determine effective dosage, and further determine the vaccine's effectiveness in this phase. Its success will enable the launch of a large-scale trial to test the effectiveness of the vaccine with the participation of up to 30,000 volunteers (Phase 3) in Israel and/or abroad, the MoD said.
IIBR started the first phase of human clinical trials on November 1 after receiving all the necessary approvals from the Ministry of Health and the Helsinki Committee. The Helsinki Committee deals with research approval and human experiments. The committee is mandated to ensure the well being and rights of experiment participants and subjects and to ensure that the research/experiment is conducted in accordance with the approved medical guidelines and ethics. These guidelines are outlined in the Declaration of Helsinki and the various international conventions concerning medical experiments on humans. It has to also take into account that the process follows laws and regulations which were legislated by the Israeli parliament and the ministry of health with the aim of regulating the issue of medical experiments on humans. The three stages of the human clinical trial involving over 30,000 volunteers are likely to last till almost mid-2021, and if all goes well the vaccine could be ready for mass use then. "We are now beginning a crucial phase [in the development of the vaccine], the clinical trials phase. I believe in the abilities of our scientists and I am confident that we can produce a safe and effective vaccine," Director of IIBR, Prof. Shmuel Shapira, had said when the trials started. "The commercial name of the vaccine is 'Brilife'. The first part of the name, 'Bri', alludes to the Hebrew word for health, 'briut', the second part, 'il' alludes to Israel, and 'life' speaks to the importance of the vaccine," Shapira said.
The vaccine developed by IIBR is based on an existing virus (VSV). Coronavirus spikes have been 'engineered' onto VSV, allowing the vaccine to attach to cells in the body. Israel's Health Ministry on Monday morning reported 1,707 new COVID-19 cases, bringing the total infection count to 357,859. The country has reported nearly 3,000 deaths so far.
Read all the Latest News, Breaking News and Coronavirus News here
















Comments
0 comment