views
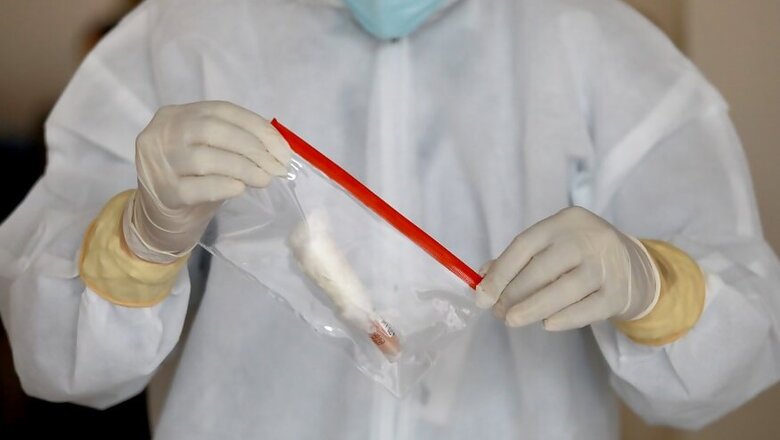
Bengaluru: While the World Health Organisation has stressed time and again that tests, more tests and even more tests are the only way to keep a check on the spread of the Covid-19 pandemic, India is caught in a controversy over its low testing and faulty imported test kits. But hopefully, not for long.
Many startups in different parts of the country have got completely indigenously-built testing technologies and capabilities validated, while a few others are in the final stages of approval. These kits will also cost just about Rs 900 to Rs 1,200 per patient, as against Rs 4,500 that is currently the cost of a test in the country. Some of these have also started manufacturing and scaling up.
"We are doing 20,000 tests per day capacity now, and could increase to 50,000 per day. We have supplied to Goa, Andhra and next Chattisgarh," says Chandrasekhar Nair of Bengaluru-based Molbio Diagnostics.
Theirs is a point-of-care test that can be deployed even in primary health centres if need be. It comes equipped with its own machine that can test anywhere between 8 to 40 samples at a given point of time.
Noida-based DNAXperts has also developed two variations of test kits — the first one is similar to the Real Time Polymerised Chain Reaction (RT-PCR) test kit that is currently used — but this company promises to cut short testing time by more than half and needs just 58 minutes as against the current 2 to 3 hours; a second one that does away with one entire process — where the RNA extraction that is part of every test process and takes about 2 hours can be by-passed. (RNA stands for ribonucleic acid, and it is the pattern of this component in the virus that will show whether a person is infected or not)
The first version was submitted to the Indian Council for Medical Research (ICMR) about a week back and the second version was submitted on Tuesday. Both await final approvals.
"Once it is approved, this reduction in testing time will have a huge impact. If we are doing, let's say, 1,000 tests per day today, we can automatically do 2,000. It will also cost only about Rs 900 and therefore far more cost effective," says Dr Ashwani Kumar Mishra, Founder, DNAXperts Pvt Ltd.
If approved now, the company can perhaps produce the capacity to have 8 to 10,000 kits per day."We should be able to scale up to one lakh kits a day, but we would need about 15 days at least to scale up," he says, adding that his company started working on developing this kit only on March 23.
India has so far depended on China or other countries purely because it was ill-prepared to manufacture anything in bulk quantity till now, he rues.
It is precisely because dependence on China or other countries during a pandemic could backfire, that an incubation centre in Bengaluru called on startups to come up with ideas.
India's Scientific Journey Set to Change
"India's scientific journey will change now. We have been usually dependent on international products. But at a time when that is not available, need is the mother of invention," says Dr Taslimarif Saiyed, CEO and Director of CCAMP, who started this initiative to bring together the best ideas and validate them from the point of view of science.
C-CAMP, the Centre for Cellular and Molecular Platforms, is a Union Department of Biotechnology centre that fosters industry-research exchanges. It was in late March that this centre launched the intiative to call upon ideas from companies and institutes for medical-research solutions to fight the Covid19 pandemic — there was only one condition that CCAMP put — that any idea or innovation suggested by anyone must be immediately deployable.
The Centre, which is vetting these ideas, has shortlisted about 25 of them — some relate to made-in-India test kits, others on disinfecting surfaces, medical equipment such as non-invasive ventilators, all of which make a big difference in treatment. It also helps process validation from regulatory bodies for the startups that have come up with the right solutions.
"We have a large innovation community which is constantly innovating for building products for a lot of things, but we were clear in our parameters — we cannot wait for five years of research, which may be needed for this certainly but doesn't serve our purpose currently. We said the innovations must be deployable soon, within at the most a month," Dr Saiyed told News18.com
One of the companies to get their stamp of approval is the Hyderabad-based Huwel Lifesciences Pvt Ltd — which has also come up with a test kit that has now got the ICMR’s approval.
Three days ago, Huwel sent out a consignment of 10,000 test kits to the Telangana government. It followed it up with two more consignments of 10,000 and 5,000 each to two other stakeholders.
The going, though, has not been easy, says Huwel's Founder-Director Shesheer Kumar. "Our test is similar to the RT-PCR kits currently being used, but what is different is, we make enzymes and reagents in-house. This is the most important component for the test," he says.
Their test costs about Rs 900 per test and takes about 1 hour and 45 minutes for each test. So it again saves on time and cost. While they started developing of a test kit in January, they got ICMR's validation in late March and then it took about 15 more days to get a manufacturing license.
“In February or March itself, they (government) should have invited all Indian manufacturers — not just us but all —and told them the requirements that would be coming up. That there was going to be a huge demand for this test. In a pandemic, it is quality that matters. You put out your criteria and tell us what to build. That is how you build the eco-system and it has not happened in Indian manufacturing," Kumar rues, adding, "I think the basic problem is, nobody knew what to do. Nobody knew. Finally, it came to a situation when things were already upon us, in the third week of March."
It is not prudent to depend on a single vendor in the time of a pandemic when every country is affected and access to raw materials is a very difficult, especially if they are to be imported, he points out.
"License also needs to be speeded up. In an emergency, you look at quality. The final objective is — if you are doing the right kit, if you doing it in India, and if your manufacturing facility can cater to volume. If these objectives are met, you should not (look for) huge set of documentation," he says.
Importing a vast number of test kits that were FDA-approved would have pushed up costs, but even assuming that some labs may have invested heavily on equipment and overheads, the cost of Rs 4,500 is pretty steep, he says.
His company's capacity is to be able to meet a target of approximately 1 lakh kits a week. If, for instance, five companies are able to do that, India's testing capacity will spiral up. "It is in the ability of, how localised can you do this. And this should help Indian manufacturing at this point of time," he says.
While these startups have developed kits that will still take about an hour for each test, there is a lab in Ahmedabad that is trying to bring down testing time to just 10 minutes. That is also a kit under development and will take about two weeks before it is validated -- but if it gets approved, the time to process each test will get reduced to a large extent. For instance, the current machine that tests 96 samples at a time takes about 120 minutes per cycle of test.... but if the same test can be done in ten minutes, you can scale up the number of samples by more than ten times.
There are other start-ups that are also working on a rapid test kit. This is not a test for someone who is a likely high-risk contact, but one that will be used for random sampling in each district or town as part of surveillance. Rapid tests can be done at home by the public themselves and will help track if there is any community transmission, but the current rapid test kits that were imported from China have proven faulty and are being sent back after they threw up inaccurate results in many places.
















Comments
0 comment