views
Slower than anticipated production of the second dose of Sputnik V, heavy cold storage requirements, pending WHO approval, rising Covid-19 cases in Russia and low demand at private hospitals have put brakes on the complete rollout of the Russian vaccine in India.
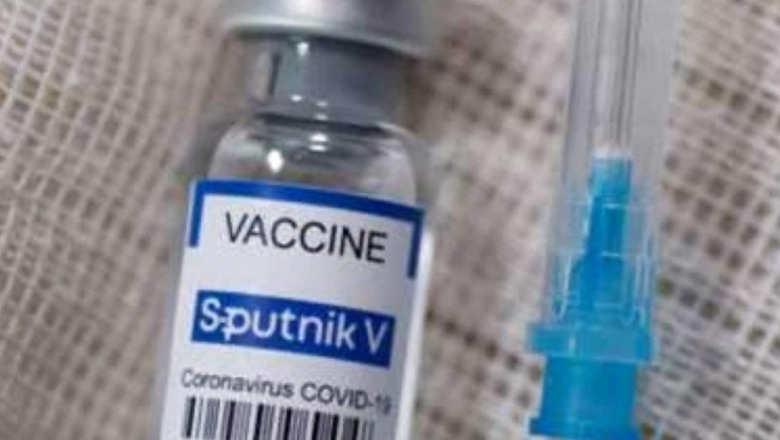
Sputnik V vaccine was first launched in India on May 1 after receiving approval from the country’s drug regulator in the second week of April.
Developed by Moscow-based Gamaleya National Research Institute of Epidemiology and Microbiology, the vaccine was registered by the Ministry of Health of Russia and became the “world’s first registered vaccine against coronavirus”.
More than six months have passed but Sputnik has not been yet commercially available across India. Out of India’s total 116 crore vaccinations, only 11.13 lakh doses were that of Sputnik V.
Till early September, drugmaker Dr. Reddy’s Laboratories — the sole distributor of Sputnik V in India under an agreement with the Russian Direct Investment Fund (RDIF) — had received around 31 lakh first dose components of Sputnik V, and about 4.5 lakh doses of the second dose component from Russia.
The “mismatch” of stock of two doses had held back the rollout of Sputnik V vaccine in India.
Vaccine experts say Sputnik V’s biggest challenge lies in the production of the second dose — the one which is termed as more “complicated” by the experts in terms of producing good yields and assembly in the laboratory.
However, the situation starting September has come under control, according to Dr Reddy’s. Thanks to the domestic production of the second dose.
News18.com spoke to Dr. Reddy’s and other experts to understand what went wrong and how it is being sorted.
Dr. Reddy’s Says ‘All is Well’ but Demand is Low in Private Market
The two-dose Sputnik V is made from two components — recombinant adenovirus vector 26 (also known as Ad26 or a prime dose) and adenovirus vector 5. Both Ad26 and Ad5 are common cold viruses affecting humans.
While Ad26 is the first dose of the vaccine, Ad5 is a second shot, which is given 21 days or three weeks apart.
According to Dr. Reddy’s spokesperson, while the two-dose Sputnik V vaccine was launched as a soft pilot in India in May, it was rolled out commercially from July based on imported consignments from the RDIF.
“We partnered with major hospitals around India, and set up our cold chain infrastructure since the vaccine requires minus 18 degrees C temperature.”
The spokesperson explained it initially received 31.5 lakh doses of dose 1 and 4.5 lakh doses of dose 2.
“In the June-August period, there were challenges with regard to the supply of the second dose component as imports were affected by the surge of Covid-19 cases in Russia.”
“In early September, with supply of the second dose component commencing from a partner in India (Panacea Biotec), our market supply received fresh momentum. We were able to supply equivalent quantities of the first and second dose components to partner hospitals.”
However, the vaccine rollout again faced a roadblock. This time, it was the falling sales at private hospitals.
“By September, in terms of the market situation, with heavy scale-up in overall vaccine production in India and free distribution through the government, private market sales overall started to see a sharp dip and Sputnik V was solely private,” spokesperson said.
Delays in Delivery of Second Dose a ‘Big Concern’
Sputnik’s biggest challenge lies in the production of the Ad5 viral vector, experts say. The poor yield of second shot is yet not resolved and it is one of the reasons why the Russian firm and Dr. Reddy’s have now shifted focus on a new single shot vaccine — Sputnik Light — which does not require the problematic second shot but only the abundantly available first dose.
A senior government official, who is a member at National Technical Advisory Group on Immunisation (NTAGI), told News18.com, “The ratio of production of the first dose to the second dose is about 5:1 as the second dose virus is slow growing. It means that only one second dose is manufactured for every 5 first doses manufactured.”
According to experts — an industry veteran, a pharma company involved in production of Sputnik V, a pharma company, which was in talks with Dr. Reddy’s to start manufacturing — the company failed to achieve expected yields of Ad5, which has affected the output, causing supply delays.
“On paper, the design is good. However, in practice, it’s manufacturability in scales is a big issue. The company promised the sky and failed,” said an industry veteran, former managing director of a top vaccine manufacturing company in India.
“In India, it entered into seven manufacturing tie-ups and only one has the vaccine experience — Panacea Biotec. Later, it went for collaboration with Serum Institute of India as well. But the output just did not happen on a scale,” he added.
While answering the questions from the media during the book launch event in late October in Geneva, Dr. Balram Bhargava, director general of the Indian Council of Medical Research, had clarified that “not crore but several Indians have been vaccinated using this (Sputnik V) vaccine.”
Bhargava had added that “the Indo-Russian collaboration, Sputnik, is being manufactured in India and sent back to Russia for exports. We are able to manufacture the first dose easily but we are still facing difficulty in manufacturing the second dose.”
Malini Aisola, co-convenor of a public health NGO, All India Drug Action Network, pointed out while quoting Bhargava’s response, and said, “There appears to have been a clear acceptance on the part of the government as well regarding the failure of Sputnik V.”
Apart from Hyderabad-based Dr. Reddy’s Laboratories, Gland Pharma, Panacea Biotec Ltd, Morepan Labs, Hetero Labs, and Serum Institute of India have been tied up to manufacture and sell Sputnik V in the domestic market as well as for export.
One of drugmakers involved in manufacturing of Sputnik V components, requesting anonymity, confessed that “everyone is facing challenges in making the second component.”
However, he added that his firm has made some quantity of second dose on “fill and finish basis from the bulk supplied,” which means that raw material was supplied and they converted it into a finished product.
Delay of Second Dose not Just India Specific
The trend of shortfall of second dose is not specific to India. Some other countries that depended on Sputnik V for inoculating their population have been pushed to look for other options due to delay in the promised timelines for delivery.
Mexico also faced delays in the supplies of the second dose apart from Argentina and several other South American countries. In fact, in the Philippines, public health bodies have suggested experimenting with mixing a first dose of Sputnik V with a second dose of AstraZeneca, under certain circumstances.
Moreover, the Covid-19 cases in Russia are going up. Sputnik V has struggled to gain acceptance inside its own country, Russia.
The mail sent to RDIF, Russia’s sovereign wealth fund, which has bankrolled Sputnik V and is in charge of marketing it abroad on November 10 and then on November 19, did not fetch any response.
According to Aisola, “A significant amount of time has passed since the production issues around Sputnik V became obvious, in spite of deflection and denial by RDIF.”
“Many countries have complained about non-delivery on orders, especially the second dose and threats of cancellation of the contracts, with some of the scathing letters sent to the Russians becoming public.”
Sputnik V is ‘Frozen Vaccine’, WHO Clearance Still Awaited
One of the disadvantages counted by the experts when compared to the available options is that it is a frozen vaccine, which requires minus 18 degrees C storage.
“It also has to be thawed to room temperature before use and has to be used within 30 minutes. The company also developed a freeze dried vaccine which can be stored at 2-8 degree Celsius but has not advanced that to scale,” said the official, an industry veteran, cited above.
The mechanism of action of the vaccine is its adenovirus, which has been modified to carry the gene expressing the spike protein of the Covid-19 virus. Once inside the body, this activates the immune system to develop the antibodies to the spike protein and thus protect against the Covid-19 infection.
In viral vector vaccines, an adenovirus serves as a “vector” or vehicle to transmit the vaccine into the body. This adenovirus is different from the Chimpanzee adenovirus of AstraZeneca vaccine, which is also made by the Serum Institute as Covishield.
While the mechanism of action is similar to Covishield but it is way stronger.
“The antigen content in Sputnik V is two times that of Covishield. It has 100 billion virus particles per dose compared to 50 billion virus particles in Covishield. This high antigen payload is an issue concerning safety of the vaccine,” said the veteran official, who is now a consultant advising vaccine manufacturing firms on production and strategies.
He further pointed out that the safety studies conducted in Russia were criticised by experts, published in well-known medical journal The Lancet, for data discrepancies and substandard reporting of interim data of phase 3 trial.
“As a result, the WHO approval for this vaccine did not materialize,” he said.
According to the WHO website, the status of Sputnik V shows “rolling submission incomplete” and “anticipated date (for decision) will be set once all data is submitted and follow-up of inspection observations completed.”
With Many Firms on Board, Vaccine Makers Suffer ‘Loss of Focus’
One of the many reasons behind the failure of Sputnik V is its strategy of “roping just too many pharma companies for production.”
“They should have stayed focussed on one or two players to start the production and then moved forward. Taking too many steps at one time confuses the entire strategy,” said the official who heads a public sector unit vaccine manufacturing firm.
“We started talks with them but they initiated the discussions with an aim to produce several millions of doses,” the official said. The talks ended on the preliminary stages only the vaccine maker was in discussions with Bharat Biotech also for production of Covaxin.
According to Aisola from AIDAN, “No company would want to take chances on large scale production of the Ad5 dose due to problems of low and also inconsistent batch yield which puts them at risk of GMP violations.”
“Companies have to maintain batch output specifications within a specified range. Wide fluctuation in batch output could become a GMP compliance issue,” she clarified.
She further said, “Even though the Russians were in the know probably before striking many of the deals with Indian manufacturers, they have been unable to provide a solution to the problems plaguing large batch production of the second dose.”
Aisola says she believes not many Indian companies are now willing to produce both doses of Sputnik V. “Since the technology transfer was offered without any strings attached and the contracts were open ended without financial obligations, deals with many of the Indian contract manufacturers could fall through.”
In September, Panacea Biotec supplied the first shipment of the second component of the Sputnik V coronavirus vaccine. The company clarified that “so far” it has not been asked to manufacture Ad26. Apart from Panacea, no other firm contracted by Dr Reddy’s has started supplying the doses.
“We have got a manufacturing license to import ready to fill bulk for both doses of vaccine and have necessary infrastructure and huge capacities to manufacture and supply both the doses of the product. We have manufactured and supplied component II (Ad5) so far to Dr. Reddy’s Laboratories (DRL) on their demand.”
“For Component I of Sputnik V, we have not received any demand from DRL hence have not made that vaccine.”
What’s Next? It is Sputnik Light
For Dr. Reddy’s and RDIF, the way forward is the one shot Sputnik Light, which is primarily the first shot (Ad26) of the already approved Sputnik V vaccine. The company has started pitching it as a “standalone vaccine” and a “booster dose” and also a vaccine for “paediatric population”.
“Going forward, we believe Sputnik continues to be relevant and meaningful in the vaccination programme,” Dr Reddy’s spokesperson said.
“Our lifecycle management of Sputnik will see us take a multi-pronged approach with the single-dose Sputnik Light vaccine (first dose of the two-dose Sputnik V) as a standalone vaccine, as a booster dose, and Sputnik V for the paediatric population.”
The company is planning to submit the approval request to the country’s drug regulator — Drug Controller General of India (DCGI) by the end of November.
“We are in the midst of clinical trials for Sputnik Light and expect to update on the status of the trial after our submission to the DCGI later this month.”
Moreover, the company is also expecting to begin export of the vaccine to other nations soon.
“We are also designing trials for the other parts of the lifecycle management of Sputnik. Finally, with the government allowance of exports, we are also in discussion with our partners to take Sputnik to other countries mostly in the Asia-Pacific region and in certain countries of Africa, Latin America and Central America.”
However, industry experts say Sputnik Light is the last bet for Sputnik V.
“Dr. Reddy’s has sunk a good amount in building cold chain distribution for a vaccine that has failed. It’s hard for them to walk away now they are so deeply invested as the party that conducted the clinical trials and has the regulatory approval in India,” Aisola said while adding that “hence the interest in pursuing approval for Sputnik Light (single dose vaccine with just Ad26) since the prospects of commercially launch Sputnik V in India were dashed.”
Read all the Latest India News here















Comments
0 comment