views
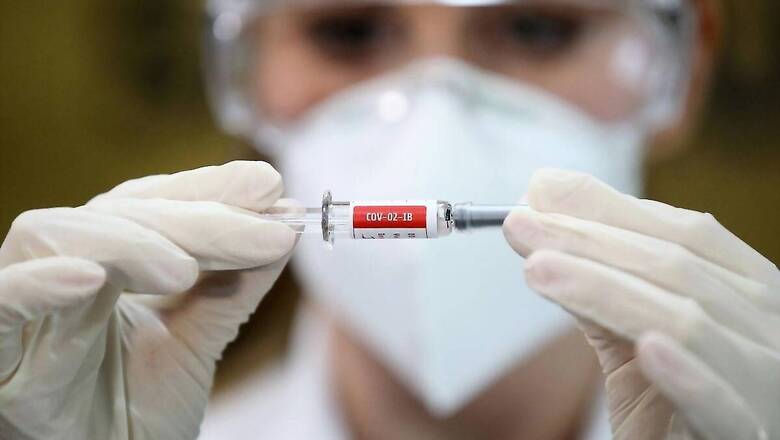
Earlier ruled out by trusted American disease expert Dr Anthony Fauci, ‘human challenge trials’ for a potential coronavirus vaccine have now failed to receive the nod of the Centre.
The human challenge trials – touted as a faster way of developing vaccines – will not be done in India, said Union Health Minister Dr Harsh Vardhan on Sunday.
“India is not planning to venture into such trials until the method is proven to have an established benefit as per global experience. The country has robust processes in place to ensure the vaccines that successfully complete the clinical trials are safe and effective against the novel coronavirus,” said Vardhan.
He said human challenge studies ought to be undertaken with abundant forethought, caution, and oversight when conducted. According to Vardhan, the “value of information to be gained” must clearly “justify” the risks to human subjects.
WHAT ARE HUMAN CHALLENGE TRIALS?
The World Health Organisation (WHO) says human challenge trials are those trials in which participants are intentionally ‘challenged’, despite whether they have been vaccinated or not, with an infectious disease organism.
The trials involve the intentional infection of research participants. The aim of such studies would be to help expedite the identification of promising vaccine candidates by providing estimates on vaccine safety, immune response as well as efficacy.
According to WHO, well-designed human challenge studies provide one of the most efficient and scientifically powerful means for testing vaccines, especially because animal models are not adequately “generalisable” to humans.
The SARS-CoV-2 challenge studies could add value to other types of vaccine research by enabling accurate assessment of asymptomatic infection; more rapid and standardised testing of multiple vaccine candidates; and testing vaccines in contexts where there is little continuing transmission – for example, due to public health measures or during inter-epidemic periods.
ETHICAL CONCERNS
During an online discussion organised by the Indian Council of Medical Research (ICMR) on July 30, Fauci, Director of the National Institute of Allergy and Infectious Diseases (NIAID) in the US, expressed concerns over conducting ‘human challenge trials’ to accelerate vaccine development for Covid-19. “Purposely exposing someone to the coronavirus is unnecessary and unethical in the case of a disease for which we have no proven cure yet,” he had said.
“Since the disease is a novel condition about which we are still learning, and for which no definitive treatment is available yet, intentional infection of volunteers in such studies also raises ethical concerns,” he had added.
Long term Covid-19 infection sequelae – a condition which is the consequence of a previous disease or injury – about what more is being learnt also accentuates such concerns,” Dr Anant Bhan, an expert in Bioethics says.
Dr Amar Jesani, Editor of the Indian Journal of Medical Ethics (IJME) and founder of the Forum for Medical Ethics Society says Human Challenge trials are usually done for infections when there is a treatment available. This, he explains, is “because if the vaccine is not going to protect you, you are going to get Covid-19 and then there ideally should be a treatment available.”
He gives the example of the trials being conducted for malaria. Experts say that much is still not known about the SARS-CoV-2, the virus that causes Covid-19 and that there is little information about the long-term effect of the infection on humans and so deliberately infecting anyone would pose an ethical difficulty.
LOGISTICAL CONCERNS
Human challenge trials have never been done in India and creating infrastructure for these studies will take time and involve huge costs, experts say. “Given that the existing vaccine candidate trials are now already in phase II/III, it is also not clear how much time would be saved by conducting said trials,” says Bhan, who is not in favour of the human challenge studies for Covid-19 “as of now”.
OTHER PROBLEMS
Human challenge trials are conducted on young and healthy volunteers. These are late stage trials, so eventually when the vaccine is developed, one will be unable to gauge if it will work on other population groups given that they were not the volunteers for the trial. If a disease as infectious as Covid-19 was being tested, the participants would also require isolation.

















Comments
0 comment