views
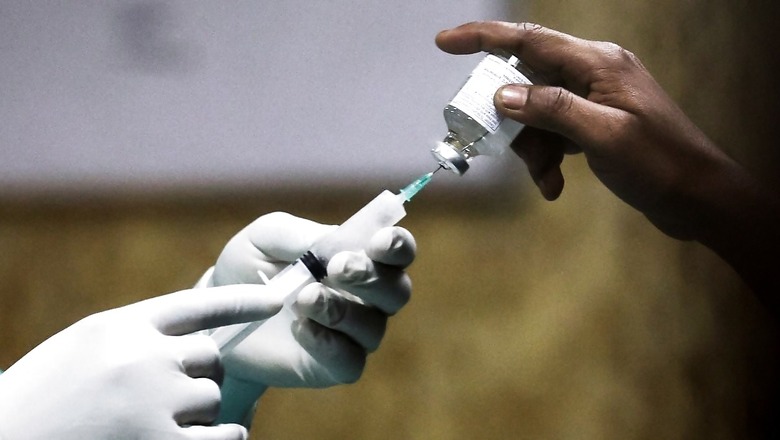
A clinical trial conducted among 303 individuals for a male contraceptive developed by the Indian Council of Medical Research showed 99 per cent potency in preventing pregnancy.
The study of the trials was published last month in a journal called Andology. “The overall efficacy of RISUG in terms of achieving azoospermia (absence of viable sperm in the semen) was 97.6 per cent. Overall efficacy based on the occurrence of pregnancies due to drug failure was 99.02 per cent,” the study was quoted as saying by the Hindustan Times.
RISUG — Reversible Inhibition of Sperm Under Guidance — is developed as a replacement for surgical method, vasectomy. This is in consideration that vasectomy is the only male sterilisation method for men in the world.
One of the lead researchers of the study, RS Sharma said that the potency of a product like this is ascertained based on two criteria, “protection against pregnancy and viability of sperms”, adding that the ICMR-developed drug has delivered excellent results in meeting these criteria. “This product has the potential to emerge as a popular modern male contraceptive for population control,” Sharma added.
According to the HT report, India was moving closer to developing the first male contraceptive injection of the world in 2019.
However, Sharma said, several projects were interrupted due to the Covid-19 pandemic and RISUG was one among them. Further studies were planned on it, he said, “Now the results have been formally published.”
The study published stated that after 21 days of getting injected, 77.2 per cent of the participants achieved azoospermia (absence of viable sperm in the semen) and 13.5 per cent showed oligozoospermia (low sperm count). Six months after taking the injection, the percentage of the subjects attaining azoospermia increased to 97.2 per cent and touched the highest level of 97.3 per cent one year after the injection.
The study was conducted at hospitals in five cities — New Delhi, Udhampur, Ludhiana, Jaipur, and Kharagpur — among those visiting family planning clinics or departments of urology or surgery for vasectomy. The Drugs Controller General India (DGCI) granted permission to conduct phase III clinical trial, also approved by the institutional ethics committee of the respective facilities.
The study found that “failure rate of RISUG is also less than that of male condom breakage, which can be as high as 12.9 per cent and 18.9 per cent (as per two different studies.” Therefore, RISUG is a one-time male contraceptive with high potency and low failure rate.
Clinical examinations have shown no serious side effects in terms of pyrexia (abnormal increase in body temperature), inflammationa at the site of injection and reproductive system, and urinary track infections.
Researchers said that “no sign of urethritis (inflammation of urethra), vesiculitis (inflammatory disease of seminal vesicles), cystitis (bladder inflamamtion), prostatitis (inflammation of prostate), epididimytis (inflammation of the coiled tube at the back of the testicle), orchitis (inflammation of testes), and dysuria (painful urination) was reported after taking RISUG infection.”
However, 41.9 per cent of the participants reported low to moderate scrotal pain on the 3rd day post the injection, which also significantly lowered in the subsequent follow up checks and 2.5 months later disappeared completely.
The sexual partners of the test subjects did not show any serious impact or side effects either during the study.
The study said that an extensive clinical examination of all female participants was done. Similar to their partners, the women also had normal baseline features at the pre-injection phase. “Female subjects also did not show any adverse side effects during the scheduled follow-up visits up to 7 years after the RISUG injection taken by their husbands.”
According to the results, a standard dose of 120 micro litre of RISUG in each vas deferens (ducts which transport sperm from the epididymis to the ejaculating ducts) accounted for variabilities in the lumen diameters along with the overall size of the ducts, the report added. “This means that the dosage do not have to be customised, making RISUG the perfect candidate for a mass contraceptive delivery programme.”
Sharma said that a few more studies are yet to be conducted, such as clinical reversibility of RISUG and post marketing supervision. For this, “we need to acquire a license to manufacture the drug at a GMP (good manufacturing practice) centre”. An application for the same has been moved to the DGCI office, he noted.














Comments
0 comment