views
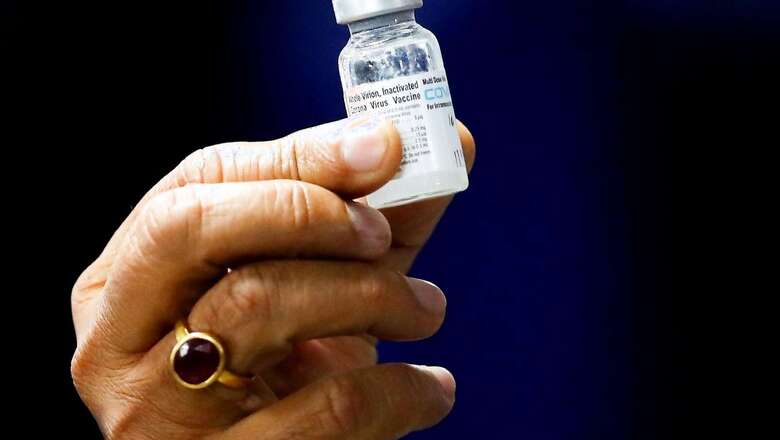
A day after India’s drug regulator approved Covovax for restricted emergency use in children aged 7 to 11 years, the vaccine maker Serum Institute has written to the Centre seeking its inclusion in the national COVID-19 vaccination programme in the interest of public health, official sources said on Wednesday. In a letter to the Union health ministry, Prakash Kumar Singh, Director, Government and Regulatory Affairs at Serum Institute of India (SII) stated that Covovax is highly immunogenic with more than 98 per cent seroconversion in 7 to less than 12 years age group.
Also, the vaccine is safe, effective and well tolerated in this age group, an official source said. According to the letter, the firm is also receiving requests from various sections of the society pan India to make vaccine available in government and private hospitals in view of increasing COVID-19 infections.
”Even employees of our firm, who have relentlessly worked on this life-saving vaccine, are requesting to us to provide this life-saving vaccine for their children soon,” Singh is learnt to have mentioned in the letter. The approval by the Drugs Controller General of India (DCGI) on Wednesday came after the subject expert committee on COVID-19 of the CSDCO last week recommended granting emergency use authorisation to Covovax for the age group of 7 to 11 years.
“The SEC last week deliberated on the EUA application of SII and recommended granting emergency use authorisation for Covovax for children aged 7 to 11 years,’’ an official source said. The expert panel in its last meeting in April had sought more data from the Pune-based firm over the application.
The DCGI had approved Covovax for restricted use in emergency situations in adults on December 28 and in the 12 to 17 years age group subject to certain conditions on March 9.
Read all the Latest News , Breaking News , watch Top Videos and Live TV here.














Comments
0 comment