views
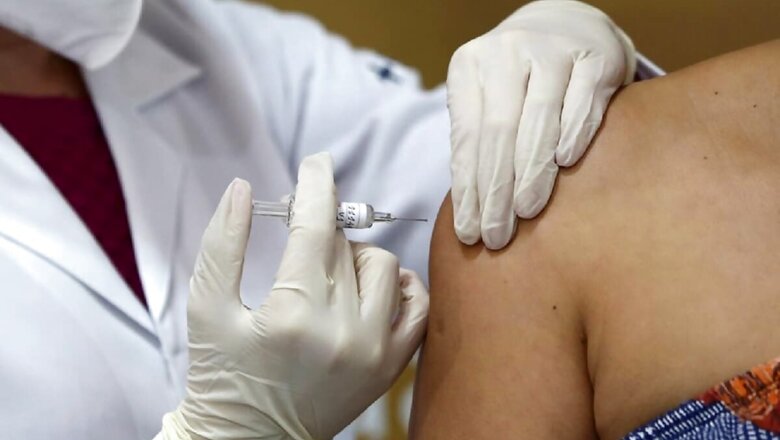
Bangladesh on Thursday gave approval for the final stage human trial of a prospective COVID-19 vaccine developed by a Chinese company, as the country reported 45 new deaths and over 2,400 fresh infections. The vaccine developed by China's Sinovac Biotech Ltd was described by the country's health minister Zahid Maleque as one of the most potential vaccine candidates in the world.
"We have given the approval for the human trial of COVID-19 vaccine developed by Sinovac in Bangladesh after examining all necessary research protocols," Maleque told a media briefing. He said the company had already got approval in Indonesia while it was trying to carry out phase-3 or last stage human trials in several other countries.
The minister said the government decided to give the final trial approval to the vaccine after the relevant government organisations examined its necessary aspects including effectiveness and safety. "We will extend all necessary cooperation to Sinovac for finishing their trial to contain the deadly virus," he said.
Bangladesh has registered 45 new fatalities from COVID-19 in a daily count, taking the total death toll to 4,127. The tally of infections climbed to 304,583 after 2,436 people tested positive for COVID-19 on Thursday, according to data released by the health directorate. Experts said the phase-3 clinical trial meant the vaccine would now be administered on thousands of people for testing its efficacy and safety.
Bangladesh's final approval for it came more than a month after the regulatory Medical Research Council (BMRC) extended the initial "ethical approval" for the prospective vaccine. The process began after Dhaka-based International Centre for Diarrheal Disease Research, Bangladesh (ICDRB) submitted a research protocol seeking to carry out the trial on behalf of the private Chinese company at nine health units dedicated for COVID-19 patients in Dhaka.
Maleque said Sinovac attached Bangladesh as a top priority country to conduct the trial. "We will get one lakh vaccines for free… and Bangladesh will get priority to purchase adequate numbers of vaccines from the Chinese company," Maleque said.
Earlier this month, Bangladesh entered into diplomatic talks with India as Foreign Secretary Harsh Vardhan Shringla made a two-day surprise visit to Dhaka when "vaccine diplomacy" appeared to have largely dominated the talks. India is collaborating with the UK's Oxford University team that developed another prospective vaccine and started its trial in the country as pharmaceutical giant AstraZeneca, which was backing the study, reported that it obtained "good data" on its COVID-19 vaccine candidate.
"Foreign Secretary Masud Bin Momen stated that Bangladesh is ready to collaborate in the development of COVID-19 vaccine (in India), including its trial, and looks forward to early affordable availability of the vaccine when it is ready," the government had said in a statement following the talks. Shringla expressed "India's willingness to be in close contact with Bangladesh and other neighbours and highlighted the cost advantage that India enjoys due to its economies of scale in manufacturing," it said.
Bin Momen said that Dhaka intended to pick one potential vaccine that would be safer and most useful for Bangladesh while "our efforts are on to get access to a potential COVID-19 vaccine". "It (vaccine) could be from China, Russia, USA or India . our discussion is underway with them all," he had told reporters ahead of his talks with his Indian counterpart.



















Comments
0 comment